The coronavirus pandemic that is hitting the world requires a war effort. On the front lines, biotech labs are working tirelessly to discover new weapons to treat and test affected patients, but also to contain the epidemic in the long term. European laboratories, which are among the best in the world, are hyperactive. There is reason for serious hope in these times of concern. An overview of the weapons under development.
The number of people affected by COVID-19 coronaviral disease continues to rise, affecting almost every country in the world. States and international health organizations alike expect tens of millions of people to be infected. A small minority of these will develop severe and potentially fatal forms. Their numbers are beginning to overwhelm medical services. There is therefore an urgent need to find a solution and to implement major measures. There should be no shortage of money, with governments and international institutions alike promising to spend heavily in order to bring the disease under control quickly.
Already, the European Commission is funding projects to develop vaccines, treatments, and diagnostics through theHorizon 2020 and the Innovative Medicines Initiative (IMI), which last week announced public grants of up to €45 million to fund studies on this disease. IMI expects pharmaceutical companies to contribute more money for a total investment of €90 million.
Charities have not been left behind in this battle. The British charity The Wellcome Trust, the Bill & Melinda Gates Foundation and Mastercard today launched a initiative 110 million to stimulate public-private collaboration and accelerate the market launch of Covid-19 treatments.
The biotechnology industry in Europe is a key element in the fight against Covid-19, in terms of vaccine development, therapeutic drugs, diagnostics and research. The organisation Labiotech, which assiduously follows European biotechnology news, has done an excellent job of identifying the initiatives of labs on coronavirus. UP' takes up this overview of the largest Covid-19 projects underway in the field of European biotechnologies.
The race for the vaccine
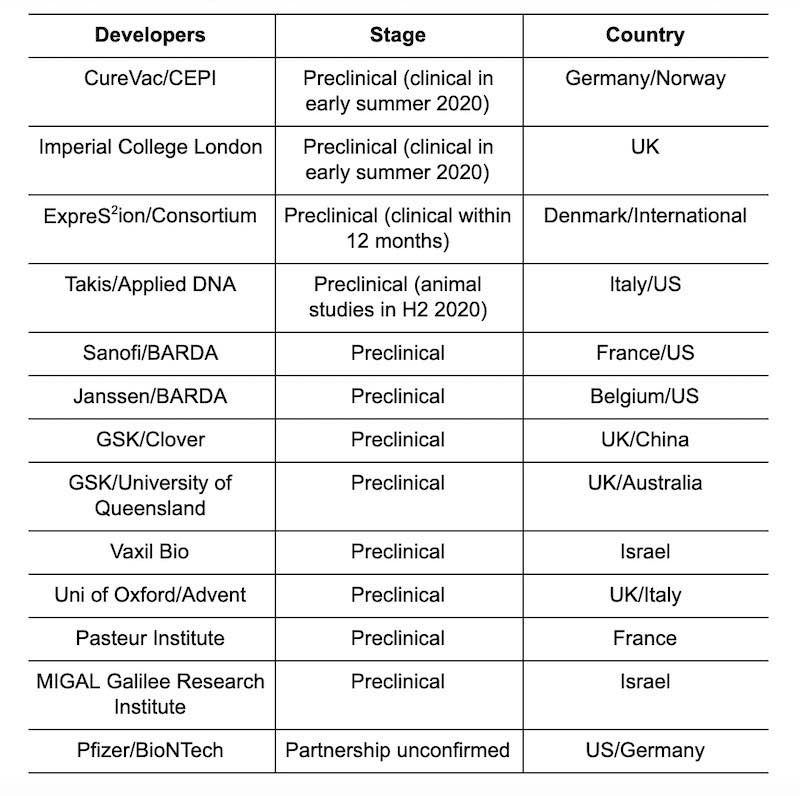
All the players in the fight to develop Covid-19 vaccines are at the preclinical stage. It is difficult to say who is winning the vaccine race, but the research supported by the major pharmaceutical companies is the one that is attracting the most attention.
For example, Sanofi and Janssen each collaborates with the U.S. Biomedical Advanced Research and Development Authority (BARDA) to develop vaccines at the preclinical stage.
One advanced program is a collaboration between the Oslo-based Coalition for Epidemic Preparedness (CEPI), funded by public and private funds, and the German biotechnology company CureVac to develop an mRNA vaccine for Covid-19. Like U.S. mRNA giant Moderna, CureVac aims to prove that mRNA vaccines will be faster to develop and manufacture than traditional biological vaccines and aims to have a Phase 1 candidate by early summer.
In addition to CureVac, the German company BioNTech - which has a number of mRNA vaccines in development for cancer and influenza - has a potential partnership with partnership with Pfizer to develop a Covid-19 vaccine.
Many other smaller projects are also underway. Yesterday, €2.7 million of the Horizon 2020 grant was allocated to a Danish public-private collaboration for the development of a vaccine against Covid-19, with the biotech companies ExpreS2ion Biotechnologies and AdaptVac. The consortium aims to start a phase 1/2a clinical trial for a vaccine within 12 months.
The Israeli Oncology Biotechnology Company Vaxil Bio is also on board, having reported proteins that could also be used as vaccine for Covid-19. There are also programs managed by the Italian company Takis Biotech, in collaboration with the American manufacturer Applied DNA Sciences, scientists at Oxford University, in collaboration with the Italian company Adventand a research group at Imperial College London, which aims to launch clinical trials in early summer if funding is secured.
Several European companies are developing methods to help other companies develop a Covid-19 vaccine. One example is the spin-off of the Danish University Immunitrack. At the beginning of March, this company published a report on some of the most promising viral structures of the coronavirus strain that could lead to a vaccine.
British society Native Antigen Company has also entered the field of vaccines by launching coronavirus antigens for research purposes, which could accelerate efforts in vaccine development and diagnostic testing.
Swiss society Roquette has a more indirect approach, developing molecules called cyclodextrins that could make vaccines more stable against Covid-19, and also make antiviral drugs easier to produce.
The first vaccines could enter Phase 1 by the summer, but regulators will likely require one to two years of testing in humans to ensure the vaccine is safe and effective. Even after approval, companies must set up commercial-scale manufacturing and distribution of the vaccine, which takes time. In total, the world could see an approved Covid-19 vaccine available by mid-2021.
Antiviral drugs
A number of companies are planning to develop new antiviral drugs or adapt existing experimental drugs to combat the new virus. Antiviral drugs are difficult to develop because, unlike bacteria, viruses hide in our own cells. This means that drugs designed to stop viruses are more likely to affect our own cells and cause side effects, such as the antiviral for the flu, Tamifluwhich can cause nausea and even hallucinations in some patients.
The Parisian company Iktos, which specializes in the discovery of drugs against avian flu, recently launched a collaboration with the American Society for Synthetic Chemistry SRI International. The objective of this collaboration is to develop new antiviral drugs to treat Covid-19, among other types of viruses.
At the end of February, the Austrian biotechnology company Apeiron launched a Phase 2 pilot clinical study of a "drug candidate" for the treatment of Covid-19. The protein-drug has already completed Phase 1 and 2 trials for the treatment of acute lung injury and is designed to work by mimicking a protein to which the coronavirus binds when it invades lung tissue.
Many efforts are also being made to redirect approved antiviral drugs to treat Covid-19. This has the advantage that the safety of the drug is already known and can be brought to market more quickly.
Scientists based at institutions in Goettingen and Berlin, Germany, are studying the potential of camostat mesilate - a drug approved in Japan for the treatment of inflammation of the pancreas - to protect against coronavirus by blocking a protein vital to the function of the virus.
Another effort to reorient antiviral drugs took place at the Norwegian University of Science and Technology. The authors of a study published in the review International Journal of Infectious Diseases identified 31 approved antiviral drugs that have the potential to treat or prevent Covid-19, such as lopinavir and ritonavir.
While research on antivirals in Europe is in its infancy, efforts are already underway to rapidly redirect approved antivirals in China, with phase 3 trials underway for treatments such as a combination of lopinavir-ritonavir and Remdesivir.
Diagnostic tests
Although the focus is on vaccines and drugs, the importance of diagnostics for Covid-19 cannot be overlooked. There is a strong global demand for rapid identification of cases of Covid-19. Their accuracy is essential to know and combat the disease and its potential for contagiousness, morbidity or lethality. The United States discovered this at its expense when official tests for the disease were found to contain defective reagents, questioning the accuracy of the U.S. disease case numbers.
In mid-February, the French diagnostics company Novacyt launched what it believes to be the first approved test to clinically detect the virus responsible for Covid-19. This follows the launch of the same test for research purposes only at the end of January. The company is now working to roll out the test in other territories.
Last week, the British biotech company Mologic received a €1.1 million grant from the UK government and the Wellcome Trust to fund the development of a portable diagnostic device that detects Covid-19 in ten minutes, without the need for a laboratory or electricity. The company is also working to manufacture the device in Africa to manage possible outbreaks on the continent.
Other companies developing tests for Covid include Qiagen, which distributed diagnostic tests for clinical evaluation at the end of February. The Finnish company Mobidiag, which is following closely, began developing a 30-minute clinical test for Covid-19 in early February in collaboration with the Chinese company Autobio Diagnostics.
In addition to diagnostics, European companies are also active in developing tests that help researchers study the coronavirus.
Austria's new generation sequencing company Ares Genetics joined the fight at the end of January, when it signed an agreement with the Chinese company BGI Group to monitor the virus and contribute to efforts to control its spread in Europe.
The German company Genekam also launched one of the first tests for the coronavirus behind Covid-19 in early February, which was intended solely for research purposes.
Even with the most accurate and rapid diagnostic tests, containment measures are also important. According to one study published yesterday in The LancetChina's policies of quarantine and social distancing can help contain the epidemic.
- READ ALSO IN UP' : What are the best current treatments for coronavirus?
Source Labiotech.eu