How many deaths will the coronavirus cause? No one is yet able to answer this question, but in the worst-case scenario, it could number in the millions around the world, warn experts, who therefore stress the importance of measures such as containment. Time is running out in the face of a galloping threat. That's why laboratories around the world are in a mad rush to find out who has an effective treatment, who has a vaccine. An unprecedented effervescence that some bad tongues say is dictated by the lure of big profits, but which can also result in the extinction of the epidemic and the recovery of millions of sick people. Things are changing by the hour on this research front. Here are the latest echoes.
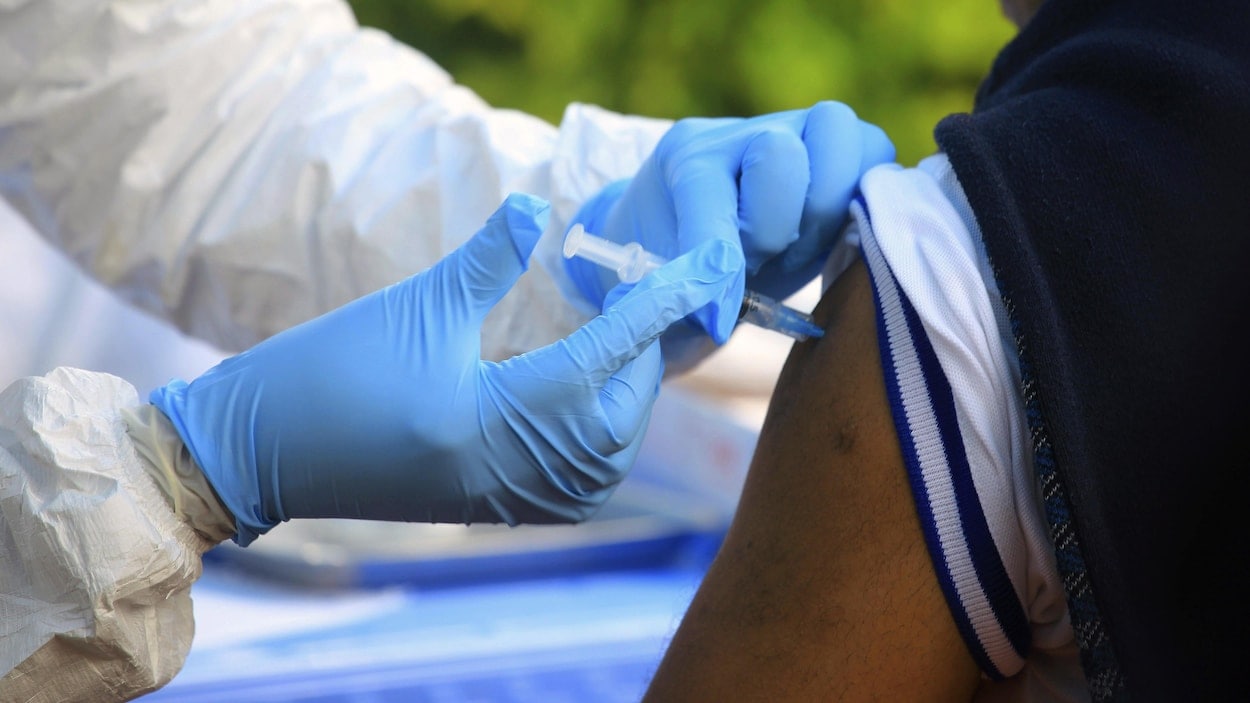
Moderna conducts first vaccine trials in the US
The first clinical trial to test a candidate vaccine against the new coronavirus began Monday, March 16 in Seattle, U.S. health officials said. The vaccine is called mRNA-1273 and was developed by scientists from the U.S. National Institutes of Health (NIH) and the biotechnology company Moderna, based in Cambridge, Massachusetts.
" The open-label trial will recruit 45 healthy adult volunteers between the ages of 18 and 55 for about six weeks," said . " The first participant received the experimental vaccine" Participants in this clinical study will still have to go through various phases to determine whether the vaccine is effective and safe.
The vaccine works with the genetic information of the part of the virus that attaches to and infects cells, spikes called spicule proteins. Coronaviruses are spherical and have spikes that protrude from their surface, giving them a crown-like appearance. The spike binds to human cells, allowing the virus to enter the body.
The candidate vaccine Moderna carries the genetic information for this spike in a substance called "messenger RNA". Injecting the spike's messenger RNA into human tissue causes it to grow inside the body, triggering an immune response without actually infecting a person with the full-blown virus.
If all goes according to plan, it could then be marketed within a year and a half, assuming the outbreak continues into the next influenza season.
The remdesivir, a treatment at the head of the pack
Of all the drugs competing to combat Covid-19, the American drug Gilead could be the first to reach the market. The latest phase of clinical trials (Phase 3) has just been launched in Asia.
The antiviral has been developed against other viruses such as Ebola (without being effective) and has not yet been approved anywhere. But it has shown promise in treating coronavirus patients in China, according to doctors, and has been used to help treat two patients in the United States and France.
" t would appear that there is no major clinical efficacy in the first Chinese cases, but it is a small number of patients. With the remdesivir, there is a decrease in viral multiplication, and this seems at this stage the most powerful of all, " said Jean-François Timsit, head of the medical resuscitation and infectious diseases department at Bichat Hospital (AP-HP), during an online conference of the College of Teachers of Intensive Care Medicine and Resuscitation, Thursday, March 12.
The remdesivir changes inside the human body to resemble one of the four building blocks of DNA, the nucleotides. When viruses replicate, they do so "quickly and a little carelessly," according to virologist Benjamin Neuman interviewed by AFP. Remdesivir could be incorporated into the virus during one of these replications. The antiviral would add unwanted mutations to the virus that could destroy it.
" At the moment there is only one drug that we think could be really effective. And that's to get him back on his feet." The World Health Organization (WHO) is the world's largest provider of health care services," Bruce Aylward, a World Health Organization (WHO) official, told a news conference. Anthony Fauci, director of the U.S. National Institute of Infectious Diseases and Donald Trump's expert on coronavirus, said the antiviral could be available "within the next few months.
The regeneron or how to make something new out of something old.
Last year, Regeneron Laboratories developed a drug, administered intravenously, known as "monoclonal antibodies", which significantly improved the survival rate of patients affected by the Ebola virus.
To apply their coronavirus treatment, the company genetically modified mice to have an immune system similar to that of humans. The mice were exposed to viruses, attenuated forms of viruses, or viral proteins, to produce human antibodies, Christos Kyratsous, vice-president of research at Regeneron, told AFP.
These antibodies are then isolated and screened to select the most effective ones, which are grown in laboratories, purified, and then administered to humans intravenously. " If all goes well, and it should, we should know what the best antibodies are in the next few weeks," and clinical trials are expected to begin this summer, according to the official.
The drug could work as both a treatment and a vaccine, by administering it to people before they are exposed, although the effects would only be temporary because the antibodies will not be part of the memory of an individual's immune system.
Regeneron is also trying to fight the lung inflammation that develops during severe forms of the new coronavirus by using another of its drugs, Kevzara, originally designed to treat inflammation due to arthritis.
Sanofi Pasteur says it is one step ahead in the race for the vaccine ...
The French pharmaceutical group Sanofi has partnered with the U.S. Department of Health to develop a candidate vaccine using "recombinant DNA technology". It consists of combining the DNA of the virus with the DNA of a harmless virus to create a new cellular entity capable of provoking an immune response. The antigens created by this process can then be replicated on a large scale.
This technology is already the basis of Sanofi's influenza vaccine. Thanks to its research work on Sras in particular, the company believes it has a "head start" in the rapid development of a vaccine against Covid-19.
David Loew, executive vice president and chief executive officer of Sanofi Pasteur, estimated that he could have a candidate vaccine "in less than six months" and potentially enter a clinical trial "in about a year to a year and a half".
... and offers France millions of doses of a promising treatment...
French government spokeswoman Sibeth Ndiaye said earlier that the clinical trials were "promising" and would be extended to more patients. These new clinical trials "
Plaquenil, a hydroxychloroquine molecule, also used for decades in autoimmune diseases such as lupus or rheumatoid arthritis, could indeed have an effect on the disappearance of the virus, said Monday, March 16 Professor Didier Raoult, director of the University Hospital Institute of Marseille. According to this study carried out by Professor Raoult on 24 patients carrying the coronavirus, six days after the start of taking Plaquenil, the virus had disappeared in three quarters of those treated.
French government spokeswoman Sibeth Ndiaye said earlier that the clinical trials were "promising" and would be extended to more patients. These new clinical trials " will be carried out with a team independent of Professor (Didier) Raoult." Ndiaye said after a Council of Ministers meeting, stressing that at this stage " we have no scientific proof "that this treatment works.
Several experts call for caution in the absence of further studies and because of the potentially serious adverse effects, especially in the case of overdose. These criticisms were swept aside by Prof. Raoult's team, who explained that hydroxychloroquine has been a drug used for many years: "Of course, there are serious side effects if you don't respect the dosage, but it's a drug that you know, you know how to do it."
Inovio pledges one million doses of vaccine by the end of the year
Inovio, a US biotech company, has been working since its creation in 1983 on DNA vaccines, which work like the other RNA-based vaccines mentioned above, but further up the chain.
DNA is like a reference book in a bookstore, and RNA is similar to a photocopy of a page of that book with instructions for performing a task. " We plan to start clinical trials in the United States in April and then quickly in China and South Korea, where the epidemic is affecting most people," said J. Joseph Kim, president of Innovio, in a statement.
" We intend to deliver one million doses by the end of the year using our existing resources and capabilities"
The German vaccine candidate that excites Trump's lust
In playground parlance, it's called wind. The German laboratory CureVac is working on a vaccine against COVID-19, like so many others around the world. But this one takes a novel approach and promises tests within a few months. That was all it took to whet the appetites of Donald Trump, who, with his usual elegance, offered to buy the vaccine exclusively from the laboratory's directors for the sole use of its American citizens.
Immediately the reaction of the German authorities resounded in the form of a colossal Nein! There is no question of letting go for the exclusive use of a nation a vaccine that could be a common good of humanity. An anecdote that one might smile if times were not so dramatic, but which says a lot about the harshness of the battle for the vaccine.
CureVac's method is original; it consists of using mRNA to develop vaccines. Classically, a vaccine consists of introducing a more or less inactivated form of a virus or a bacterium into the organism, so that the organism can organise its defences without the risks that a real infection would entail. At CureVac, researchers are working on the opposite strategy: instead of luring the body with a fake virus so that it is ready to receive the real one, they directly arm the body's cells to deal with the real virus. To do this, they use messenger RNA, or mRNA, the molecule that acts as an intermediary between the DNA and the proteins it encodes like a real cookbook. Each mRNA guides the synthesis of a specific protein. By introducing an mRNA made specifically to give rise to a protein designed to be effective against an infection, it is therefore theoretically possible to obtain immunisation.
It is on this principle that researchers at CureVac have been working on a vaccine against Covid-19 since the end of January 2020, in partnership with the public organization CEPI (Coalition for Epidemic Preparedness Innovations). Thanks to this additional funding, the company promises accelerated development, so that it will have a candidate vaccine to test on humans "in a few months", according to Richard Hatchett, CEO of CEPI, in a press release. " We are currently developing a vaccine that, after successful preclinical testing, could be tested rapidly in humans in a clinical study," confirms Dr. Mariola Fotin-Mleczek, CureVac's Chief Technology Officer.
If CureVac is successful, millions of doses of vaccine could potentially be produced at low cost in its existing production facilities.
Urgency and Caution
This race for coronavirus treatments and vaccines is dictated by the urgency of the situation. Morbidity and mortality curves are climbing in all countries, promising dizzying heights. Faced with this pressure, laboratories are accelerating and are massively supported by their respective authorities in their efforts.
But this acceleration of the search quickly finds points of friction and slowing down. They are due to the legitimately cautious marketing rules, requiring heavy and above all very long test protocols. It has taken many years to put in place an elaborate framework to ensure that clinical research is carried out ethically and scientifically.
Today, however, the pressure of the emergency response to the coronavirus crisis is such that a decision may have to be made to amend the rules and consider whether it is appropriate to accept higher levels of risk to research participants and patients. Will we need to find ways to reduce bureaucracy and over-caution in order to speed up decision-making and make the system more responsive? This is a serious question to which all of us, patients, doctors, health authorities and states alike, will be in a hurry to find an answer.
- To go further: a state-of-the-art scientific article on vaccines and treatments for VIDOC-19 by Fatima Amanat and Florian Krammer : SARS-CoV-2 vaccines: status report (pdf)

With AFP